5.2. The process panel
By using the options provided on the process panel, you may control the chemical modification rates, filter settings and stereo chemistry.
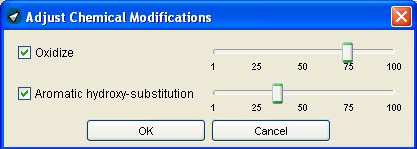
5.2.1. The chemical modification rates
The chemical modification rates are used to define the rate of oxidizations, aromatic hydroxy-substitutions and aromatic amino-substitutions. These three features contribute to the diversity of generated molecules. The chemical modification rate increases exponentially: While a 40% chemical modification rate means that only about eight out of 100 modifiable atoms are altered, a 90% chemical modification rate allows modification of nearly every modifiable atom.
The oxidization rate
The oxidization scale allows you to adjust the rate of oxidizations of generated molecules. High oxidization rate means more oxidized chemical features and more keto-groups added to the compound.
The aromatic hydroxy-substitution rate
The aromatic hydroxy-substitution rate defines the ratio of aromatic hydroxy substitution. This is a powerful feature to increase drug-likeness and diversity of the generated molecules containing aromates.
5.2.2. The filters
The filter module is a powerful tool to select the physicochemical properties of generated compounds. For detailed instructions see chapter 6.
5.2.3. Stereo chemistry settings
The stereo chemistry module allows to define steric properties of generated molecules.
Activate the stereo chemistry checkbox to enable the drop-down menu and the customize field. The drop-down menu contains three property settings for stereo chemistry: Assign mixed, custom and no R/S centers.
Assign mixed generates racemates of chiral molecules and compounds with cis/trans isomerism (i.e 50% of all chiral atoms in R respectively S configuration and 50% of all double bonds in cis respectively trans configuration).
No R/S centers eliminates all compounds with R/S centers.
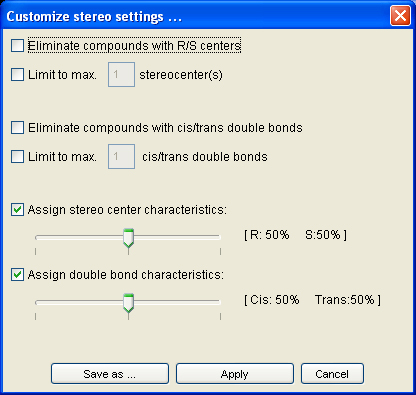
The following features can be adjusted/selected in the stereo chemistry module:
- Elimination of compounds with R/S centers
- Limitation to max. stereocenters
- Elimination of compounds with cis/trans double bonds
- Limitation to max. cis/trans double bonds
- Ratio of stereo center characteristics
- Ratio of double bond characteristics
Currently double bond characteristics cannot be exported or viewed when generating 3D coordinates with CORINA.
