Example 3: Generation of a Combinatorial Library Employing the Biginelli Multicomponent Condensation
Introduction
Multicomponent reactions (MCRs), like the Biginelli, Mannich, Passerini, and Ugi reactions, are single-pot reactions of three or more reactants which form products containing portions of all the components. Although some of these protocols have been known for over 100 years, MCRs have recently found new popularity with their implementation in combinatorial and solid-phase synthesis, allowing the generation of large combinatorial libraries in a single step. The Biginelli condensation is a versatile reaction for the generation of substituted dihydropyrimidinones [1]. In this example, we take a recent publication on this topic and translate the chemical reaction into a protocol for ilib diverse. This allows us to reproduce a subset of the products which are accessible from the educts reported in the paper [2].
Chemical Background
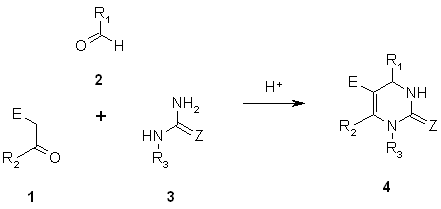
As shown in Figure 1, the Biginelli reaction is an acid-catalyzed condensation of a CH-acidic carbonyl compound 1, an aldehyde 2, and urea 3, leading to the formation of 1,4,5,6-substituted dihydropyrimidinones 4 (DHPM). In the reference paper [2], 17 different CH-acidic carbonyl compounds (1A-Q), 25 different aldehydes (2a-y), and eight different ureas (3 alpha- phi) were used. For our example, we selected all of the CH-acidic carbonyl compounds and aldehydes and the four substituted ureas beta- epsilon (see original paper for details).
Figure 1: General reaction scheme for the Biginelli condensation. R-3 = H, alkyl, (substituted) aryl, ...; E = electron-withdrawing group (ester, amide, nitro, ...); Z = O, S.
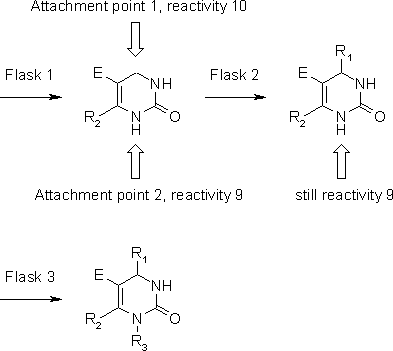
Library Generation with ilib diverse
Figure 2 describes the general procedure for the construction of our target library. We choose the DHPM core structure as the starting fragment, to which we then attach our substituents. Since there are only 17 possible combinations of R2 and E, predetermined by the 17 CH-acidic compounds 1A-Q, the 17 possible substituted DHPM core structures resulting from these compounds have to be drawn and are imported into ilib diverse, to the new group "Biginelli - DHPM core with R2 and E". Next, the residues for R1, originating from aldehydes 2a-y, are drawn and imported into group "Biginelli - R1". Although some of the fragments are already present in the default fragment set, we choose to include all the necessary fragments in their appropriate groups. The same is done with fragments representing R3, which are added to the group "Biginelli - R3". Reactivities are set in the edit atom properties window for each of the imported fragments: At the DHPM compounds, reactivity 10 is set at position 4 (attachment point 1, Figure 2), selecting reset reactivity after first substitution , to prevent disubstitution at this position. Additionally, reactivity is set to 9 at position 1 (attachment point 2). Now the reactivities of the fragments from the two other groups are modified, always setting a value of 10 at the position where substitution should occur, (i.e. the position, where the carbonyl group or the urea nitrogen are attached in the original compounds), except for those fragments, where there is either only one possible attachment point (methyl), or where all possible attachments yield the same results (unsubstituted phenyl, ethyl). Again, reset reactivity after first substitution is selected at positions where disubstitution is possible.
Figure 2: Construction of a Biginelli library with ilib diverse.
We now select the button Ordered groups, set the number of fragments to 3, and place group "Biginelli - DHPM R2 & E" into flask 1, group "Biginelli - R1" into flask 2, and "Biginelli - R3" into flask 3, leaving all weight values at 100%. Filter properties are deselected, and Stereo Chemistry is set to "Assign Mixed". With this, the library preparation is complete, and 17*25*4 = 1700 synthetically accessible compounds, each as either R or S enantiomer, can be generated.
Notes / Other Things You Could Try
- In the current version of ilib diverse, it is not possible to select an "empty" fragment, providing the possibility of leaving a position unsubstituted. Thus, to generate compounds for which R3=H (corresponding to unsubstituted urea 3 alpha from Ref. [2]), you have to reduce the number of fragments/flasks to two, leaving away the last group.
- To generate the thio-urea compounds generated from thioureas 3 theta, eta, and phi described in the reference, replace the oxygen at position 2 with a sulfur atom for all the 17 DHPM core structures in a molecule editor of your choice, use methyl and phenyl as substituents in R3, and, again, remove flask 3 to get the N1-unsubstituted thioanalogues theta.
- To get a larger collection of possible Biginelli compounds which is not restricted to the compounds described in the reference, you can do the following: Draw and import the unsubstituted DHPM core structure and its thio-analogue, and put them into flask 1. Set different reactivities at positions 1, 4, 5, and 6 of the DHPM structures. Put/import different fragments into flasks 2-5, corresponding to residues R1, R2, R3 (alkyl, aryl, ...), and E (esters, amides, nitro, ...). You can either set higher reactivities at the desired attachment points for the groups in flasks 2-5, or leave the reactivities at their standard values, to allow a larger variety of generated compounds.
- Try to find a similar procedure for other multicomponent reactions, such as the Ugi four-component reaction (U-4CR).
References
[1] Biginelli, P. Gazz. Chim. Ital. 1893, 23, 360.
[2] Stadler A., Kappe C.O. Automated Library Generation Using
Sequential Microwave-Assisted Chemistry. Application toward the
Biginelli Multicomponent Condensation. J. Comb. Chem. 2001, 3(6),
624-30.
To use the fragment set and flask settings described here, import the file biginelli.ifs from the examples directory.
