Example 1: Focused Virtual Library Generation Setup for Ligands of the Human Rhinovirus Coat Protein
Introduction and Pharmacological Background
Rhinoviruses belong to the group of picornaviruses and present the most frequent cause for common cold. Antirhinoviral compounds, the so-called human rhinovirus (HRV) coat protein inhibitors, have been developed to bind into a hydrophobic pocket in the viral capsid, stabilizing the capsid and interfering with cell attachment and/or uncoating of the viruses. In this context, the WIN compounds, synthesized by the former Winthrop-Sterling group form an interesting class of inhibitors. One of these compounds, Pleconaril (WIN 63843), is expected to be launched in summer 2004 [1]. More than 100 rhinovirus serotypes are known and can be classified into major group viruses using ICAM-1 as their cellular receptor and in minor group viruses, which use members of the low-density lipoprotein family to attach to cells [2]. In the absence of an inhibitor the hydrophobic binding pocket, which is situated underneath a depression on the virus capsid surface, can be either empty or occupied by a cellular pocket factor, a lipid or fatty acid. The Brookhaven Protein Databank contains more than 30 entries of HRV coat protein in complex with an inhibitor, which allows detailed analysis of protein-ligand interactions and systematic development of novel inhibitor structures. This study is targeted on generating libraries of possible new HRV coat protein inhibitors built in an analogous structural manner as the WIN compounds using the software ilib diverse.
Template Molecules for Focused Library Generation
Highly efficacious WIN compounds generally contain three (aromatic) rings: Ring A, which usually occupies the innermost part of the binding pocket,is linked to a phenoxy moiety (ring B) via a single bond. Ring C, an isoxazole ring, is separated from this phenoxy group by an aliphatic chain of various length [3], see Figure 1. Entrance into this hydrophobic pocket through a narrow pore on the capsid surface requires a certain amount of flexibility of the compounds. Furthermore large ring systems are prohibited because of steric restrictions of the tunnel-shaped binding site. Studies on the characteristic structural framework of several WIN compounds (Table 1) guided parameter setting and the following generation process in ilib diverse.
Figure 1: General structure of WIN compounds
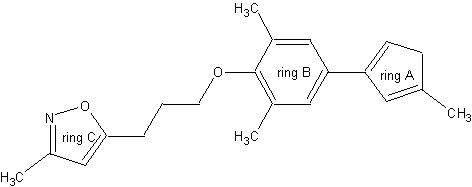
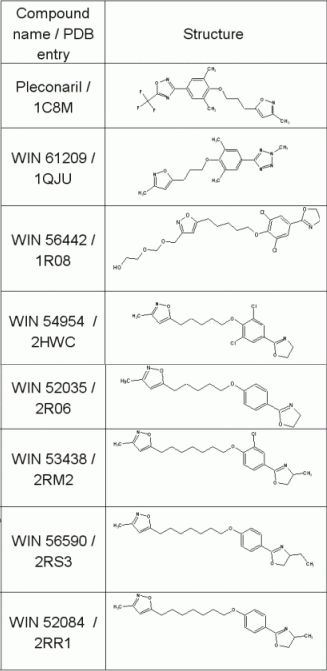
Table 1: Structures of WIN compounds used as templates for virtual combinatorial library design of potential new HRV coat protein inhibitors in ilib diverse
Library Generation with ilib diverse
For exhaustive representation of WIN characteristics, the ordered group modus is selected and a number of 6 flasks is defined for the library generation process. Figure 2 shows the important sections of a WIN molecule
Figure 2: Division of WIN compounds into six building blocks used for flask definition in ilib diverse
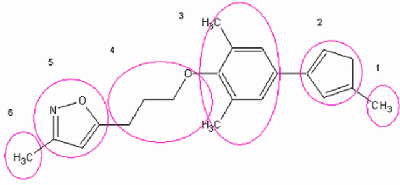
Flask 1: This substituent filling the innermost part of the binding pocket is usually composed of a small rather lipophilic group. Our fragment selection includes Methane and Ethane contained in the Aliphatics fragment set, Fluoride from the Halogen, and Trifluoromethyl from the Fx groups. Since a methyl residue can be observed most frequently in the WIN compounds at this position, the Methane fragment was given a weight of 100%. Trifluoromethyl, which is present in the most beneficial of these inhibitors, Pleconaril, was provided with a weight of 50%, whereas the other weight values were set to 20%.
Flask 2: This moiety consists of aromatic or semi-aromatic 5-membered heterocyclic rings. The ring at this position presents a less conserved, modifiable component of the structural pattern of WIN compounds. We include therefore several 5-membered rings from the Heterocycles fragment set into this flask: 1,2,4-Triazole, 1,3,4-Thiadiazole, 1,3-Thiazole, Furane, Isothiazole, Oxazole, Pyrazole, Pyrrole, Tetrazole, and Thiophene. Commonly observed at this position is the Oxazole, which is therefore given a weight of 100%. Reactivity settings of 10 and 5 for the carbons in position 2 and 4 respectively are used to guide the generation process into the desired direction. A weight of 100% is adequate for another favourable heterocycle, the Tetrazole ring system, contained for example in the highly active compound WIN 61209. Reactivity values for the other fragments remain at their default values, and their weights are set to 20%. Furthermore, a dihydrooxazole ring, which is part of many WIN compounds but is not included in the default fragments, was previously imported into ilib diverse. Due to its frequent occurrence in known inhibitors, the weight is set to 100%. A reactivity value of 10 at the carbon atom in position 4 causing a connection with flask 1 fragments there, as well as a reactivity of 5 for the carbon atom between the oxygen and the nitrogen as origin for further substitution ensure WIN analogy. Thereby, to prevent a second substitution in position 4 the ‘reset reactivity after first substitution’ command is checked for this carbon atom. Furthermore, reactivity at the C5 atom is given a value of -2.
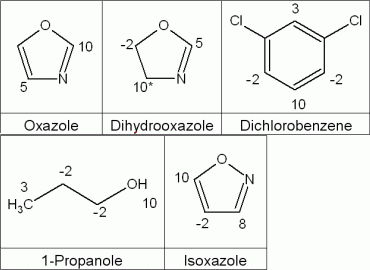
Examples of utilised reactivity settings for fragments of different flasks are displayed in Figure 3.
Flask 3: For this methyl-, chloride-, or non-substituted benzene moiety, a set of predominantly newly created fragments is selected: Benzene, Chlorobenzene, Dichlorobenzene, Chloromethylbenzene, Toluene, Dimethylbenzene, Fluorobenzene, Difluorobenzene, and Fluoromethylbenzene. Fluoride substitution cannot be found in the template molecules, although it seems an obvious choice because of its size and related chemical properties. Therefore fluorine containing fragments are only given low probability of usage for compound generation by setting a low weight definition for these fragments. Reactivity values for the benzene ring atoms at position 2 and 4, respectively, are increased (e.g. 3 and 10). Other possible starting points for substitution are prohibited by the setting of negative reactivity values.
Flask 4: An aliphatic chain of 4 to 8 atoms of length creates a linkage to the isoxazole ring situated rather at the pocket entrance region. Therefore C3 to C7 saturated, linear alcohols are selected. Reactivity of the oxygen atom as increased to 10 in order to enforce the by default prevented substitution and consequently resulting ether generation at this position. The terminal carbon atom of the chain, the desired target for the second substitution process, is set to a reactivity value of 3, whereas other atoms of the chain are protected from reaction by negative reactivity values. These alcohols are given highest weights. We also include alcohols with low arborisation, like 2-Pentanole and 2-Butanole, as fragments but with low probability of being selected for the generation. Reactivity for them is analogously defined as for the other alcohols.
Flask 5: The isoxazole ring, which is known from X-ray structure information to enable favourable hydrophobic as well as polar interactions with the binding site, is typical of the WIN compounds. Maximum reactivity is defined at position 5 of this ring system. The reactive site for flask 6 compounds is the C3 atom. Its reactivity is set to 8 in order to prevent in the majority of cases flask 6 fragments to be connected with the terminal carbon of the aliphatic alcohol chain of flask 4 alcohols (reactivity of 3) instead of with the Isoxazole C3 atom. Such an undesired substitution pattern would result in a chiral, 4-times substituted carbon atom, which would prevent the creation of molecules that are able to acquire long-stretched configurations fitting the pocket shape.
Flask 6: The last building block filling the entrance region of the pocket consists of a methyl group in most of the known WIN compounds, giving the Methane fragment highest weighting. Nevertheless, since the binding site also offers room for larger-sized residues, other fragment from the Aliphatics group, like Ethane, Propane, and Butane, several alcohols (1-Heptanol, 1-Hexanol, 1-Pentanol, 2-Propanol, Butanol), as well as an imported fragment, the side chain observed in WIN 56442, called Diether, are included into flask 6. Differing reactivities for these alcohols as compared to those used for flask 4 are needed, since in this case, substitution was desired not at the oxygen atom but only at the last carbon atom of the chain. ilib diverse does not support various parameter settings for the same fragment in different flasks. Therefore the alcohols have to be created once more, imported into ilib diverse, and saved under a different name. All other positions are protected from reaction with reactivity values of -2.
Figure 3: Examples of reactivity settings for fragments used in the generation process
*reset reactivity after first substitution
Filter Settings
Application of the ‘Lipinski rule of five’ filter ensures high drug-likeness and oral bioavailability of the generated compounds. Stereochemistry setting is defined as by default (assign mixed stereo chemistry), but no chemical modification was desired because of the high specificity of the fragment definition.
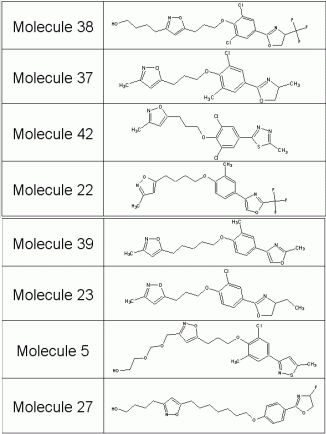
This generation process results in a drug-like library of compounds that share the same shape and the same functions as known WIN molecules. They are therefore promising candidates for synthesis and biological testing. On standard hardware, time consumption for each generated compound came up to 194ms. Out of the generation process, more than 80% of the structures did not match the filter requirements, especially the desired estimated log P, and were therefore rejected. The Report Section provides information on the generation process as well as on the estimated log P and molecular weight distribution. Examples of generated compounds are given in Table 2.
Table 2: Examples of generated compounds
References
[1] www.viopharma.com
[2] Kotalar, P.R., Bella, J., Olson, N.H., Baker, T.S., Rossmann, M.G.
Structural studies of two rhinovirus serotypes complexed with fragments
of their cellular receptor. The EMBO Journal, 1999, 18 (22), 6249-59
[3] Hadfield, A.T., Diana, G.D., Rossmann, M.G. Analysis of three
structurally related antiviral compounds in complex with human
rhinovirus 16. PNAS, 1999, 96 (26), 14730-35
To use the fragment set and flask settings described here, import the file winlibrary.ifs from the examples directory.
