Example 5: Potential Inhibitors of PKB
Introduction and Pharmacological Background
PKB plays a key role in cell differentiation, proliferation and tumor genesis. PKB is an anti-apoptotic protein kinase that has elevated activity in a number of human tumors [1]. In this context, PKB inhibitors have been demonstrated to be potent agents against cancer. Therefore, a new group of PKB inhibitors, “Akstatins” are considered as potentially attractive drugs for the inhibition of PKB. [1]
Template Molecules
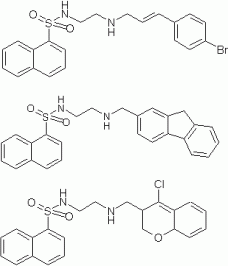
Akstatins are a group of PKB inhibitors which were designed starting from structure of the selective commercial PKA inhibitor H89.
Figure 1: Some template molecules derived from H-89
Library Generation with ilib diverse
Based on the structure of the template molecule H89, we defined three flasks for the library generation process (referred as Group A, Group B, and Group C, see Figure 2)
Figure 2: Division of template molecule H89 into building blocks used for flask definition in ilib diverse
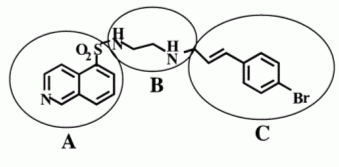
Flask 1 (Group A): The core 5-substituted isoqinoline moiety can be replaced with various bicyclic aromatic residues. The sulfonamide group can be replaced with carbonyl and other bio-isosteric moeities.
Flask 2 (Group B): The alkyl diamine bridge can vary in length, hydrogen bonding properties and substitution. Appropriate fragments have been placed into the ilib diverse group Group B. In order to assure substitution at both ends of the diamino fragments, the reactivity was set to a high value for both terminal N atoms; The Reset reactivity after first substitution flag was enabled in order to prevent the formation of tertiary amines or secundary amides or sulfonamides, respectively.
Flask 3 (Group C):The cinnamoyl moiety can be modiefied by a large variety of structures to optimize the properties of this region.
Recommended Filter Settings
Application of the ‘Lipinski rule of five’ filter ensure high drug-likeness and oral bioavailability of the generated compounds. Stereo chemistry setting is defined as by default (assign mixed stereo chemistry). This generation process results in a drug-like library of compounds that share the same shape and the same functions as PKB inhibtors. They are therefore promising candidates for synthesis and biological testing.
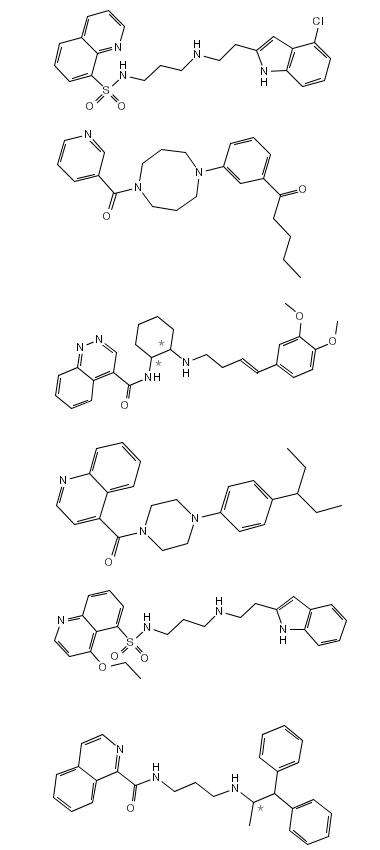
Examples of generated compounds are given in Figure 3. The Report Section provides information on the generation process as well as on the estimated log P and molecular weight distribution.
Figure 3: Examples of generated structures
References
[1] Reuveni, H. et al., Toward a PKB Inhibitor: Modification of a Selective PKA Inhibitor by Rational Design, Biochemistry 2002, 41, 10304-10314.
To use the fragment set and flask settings described here, import the file pkb-i.ifs from the examples directory.
